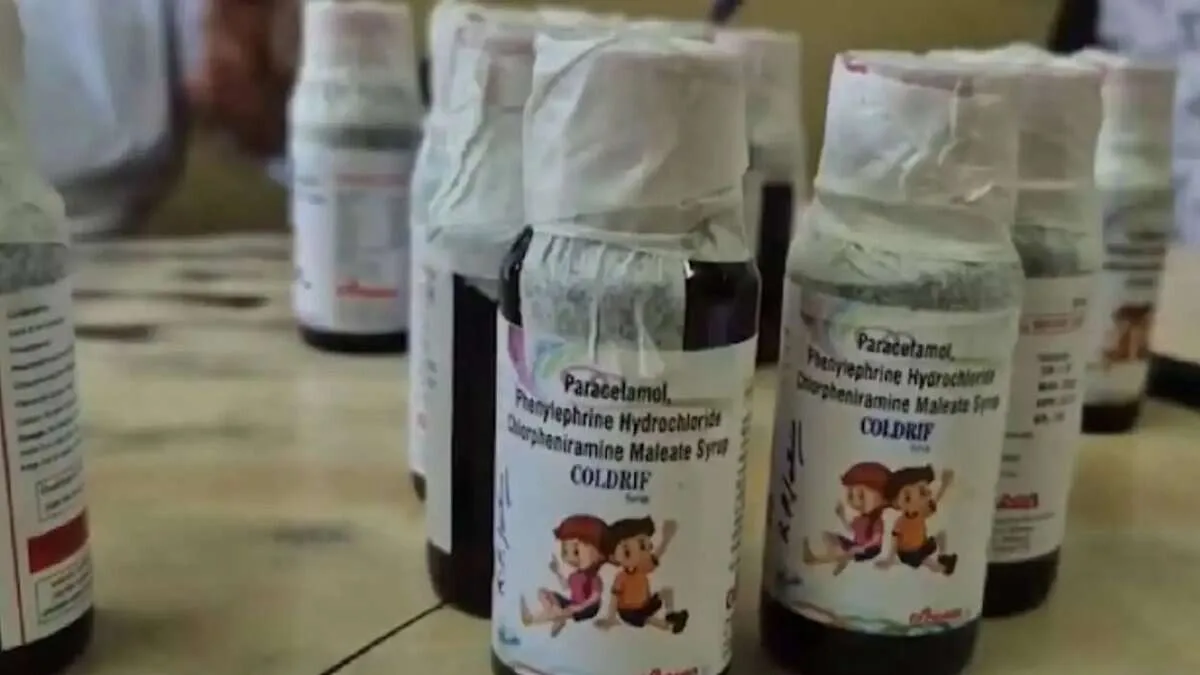
Hyderabad: The Telangana government has directed all District Medical and Health Officers (DM&HOs) to sensitise public on the ‘alert’ concerning the Coldrif cough syrup, which has been found “adulterated” with a toxic substance.
In a communication to the DM&HOs, the Telangana government’s Director of Public Health directed the officials to raise public awareness about the alert issued on September 4 by the state Drugs Control Administration to stop using Coldrif syrup of Batch No. SR-13, which has been allegedly found adulterated with Diethylene Glycol (DEG), a toxic substance.
If people are in possession of the syrup, they should immediately report to the local drug control authority on its toll free number, the communication (dated October 5), shared with the media on Monday, said.
The Director of Public Health also asked DM&HOs to strictly implement and disseminate the advisory issued by the Directorate General of Health Services (DGHS), Union Ministry of Health, regarding the judicious prescribing and dispensing of cough syrups for children.
The DGHS, in the advisory, stated that most acute cough illnesses in children are self-limiting and often resolve without pharmacological intervention.
Cough and cold medications should not be prescribed or dispensed to children under two years. They are generally not recommended for ages below five years, and above that, any use should follow careful clinical evaluation with close supervision and strict adherence to appropriate dosing and other relevant precautions.
The advisory also said all healthcare facilities and clinical establishments must ensure procurement and dispensing of products manufactured under Good Manufacturing Practices and formulated with pharmaceutical-grade excipients.
The Madhya Pradesh government on October 4 banned the sale of Coldrif syrup following the death of nine children due to suspected kidney infection in Chhindwara district since September 7.